This global, randomized, double-blind, placebo-controlled, multicenter, Phase 2 study (NCT03041311) assessed the potential of trilaciclib to reduce the incidence and consequences of chemotherapy-induced myelosuppression in patients with newly diagnosed extensive-stage small-cell lung cancer (ES-SCLC) treated with etoposide, carboplatin and atezolizumab (a programmed death-ligand 1 inhibitor).
Source: G1 Therapeutics
Patient Eligibility
- Aged ≥ 18 years
- Chemotherapy and checkpoint inhibitor naïve
- Histologically or cytologically confirmed ES-SCLC with measurable disease
- Eastern Cooperative Oncology Group Performance Status 0–2
- Adequate organ function
- Hemoglobin ≥ 9.0 g/dL
- Absolute neutrophil count ≥ 1.5 × 109 L
- Platelet count ≥ 100 × 109 L
- No symptomatic brain metastases
- No active, known, or suspected autoimmune disease that required systemic treatment in the past 2 years
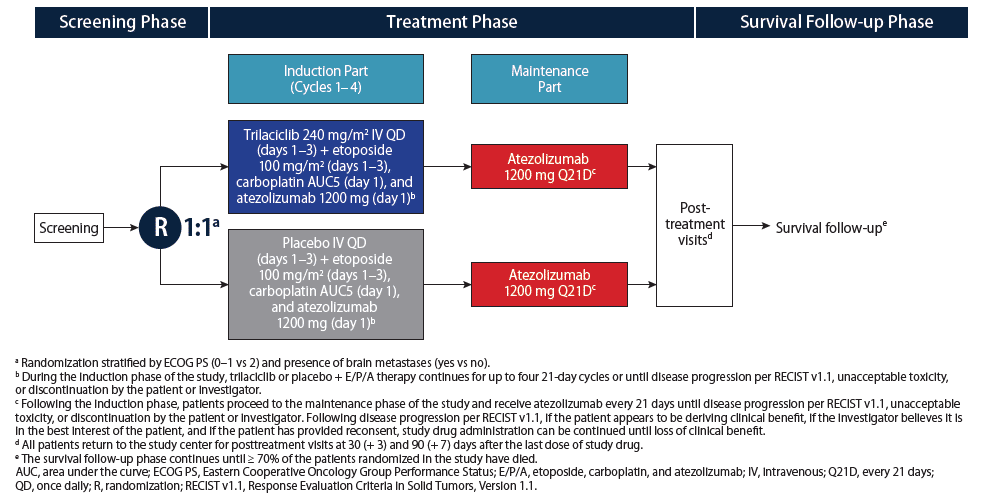
Study Design
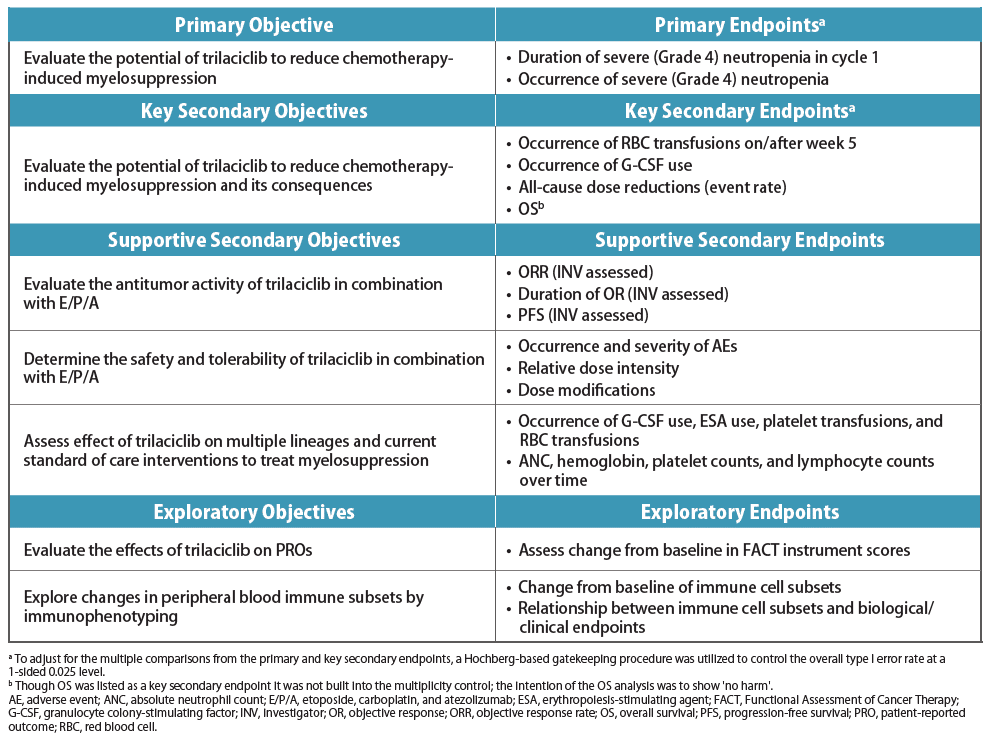
- The study schematic is shown in Figure 2. The study objectives and endpoints are shown in Table 1
- Primary prophylactic hematopoietic growth factors were prohibited in cycle 1; otherwise, standard supportive care was allowed throughout the study
- Data presented are from the following data cuts: Aug 17, 2018 for final myelosuppression endpoints (ie, after all patients had the opportunity to receive 4 cycles of induction therapy) and Aug 05, 2019 for all other endpoints
Source: G1 Therapeutics
Assessments
- Complete blood counts were obtained on days 1, 3, 8, and 15 of each cycle of trilaciclib/placebo plus etoposide, carboplatin, and atezolizumab (induction) or atezolizumab (maintenance)
- Adverse events (AEs) were graded according to the National Cancer Institute’s Common Terminology Criteria for AEs version 4.03
- Patient-reported outcomes were assessed using Functional Assessment of Cancer Therapy-Lung (FACT-L) and Functional Assessment of Cancer Therapy-Anemia (FACT-An) instruments, administered to patients on day 1 of each cycle and at posttreatment visits
- Tumor response was assessed per Response Evaluation Criteria in Solid Tumors version 1.1
Results
Myelopreservation
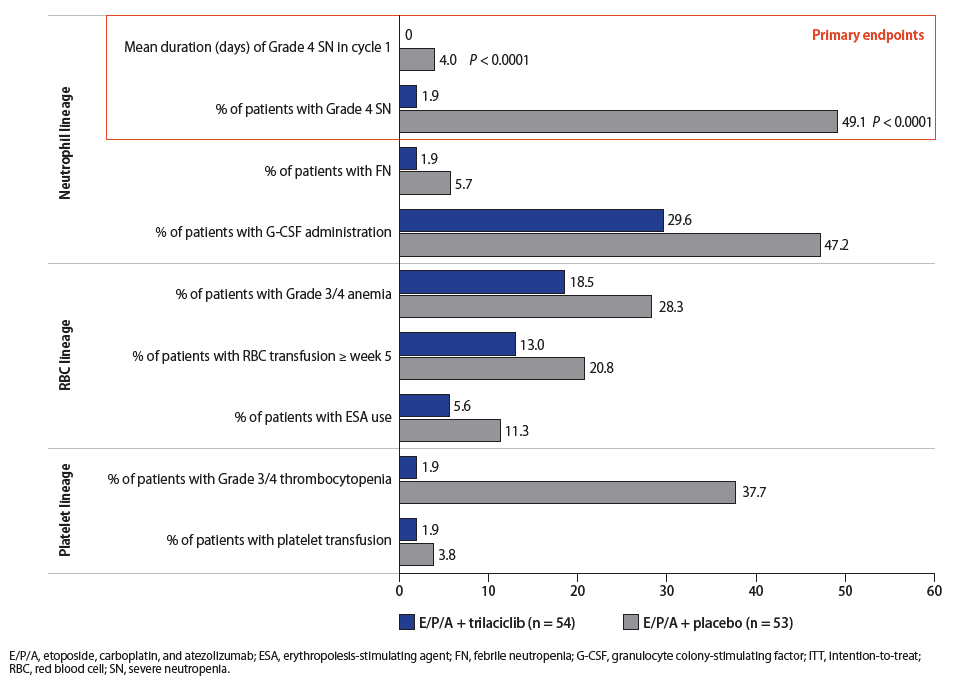
- Trilaciclib reduced both the duration of severe neutropenia in cycle 1 (P < 0.0001), a surrogate for febrile neutropenia and infections, and the occurrence of severe neutropenia (P < 0.0001), compared with placebo (Figure 3)
- Although not statistically significant, trilaciclib also reduced the need for red blood cell (RBC) and platelet transfusions and the use of granulocyte colony-stimulating factor and erythropoiesis-stimulating agents, compared with placebo (Figure 3)
Safety
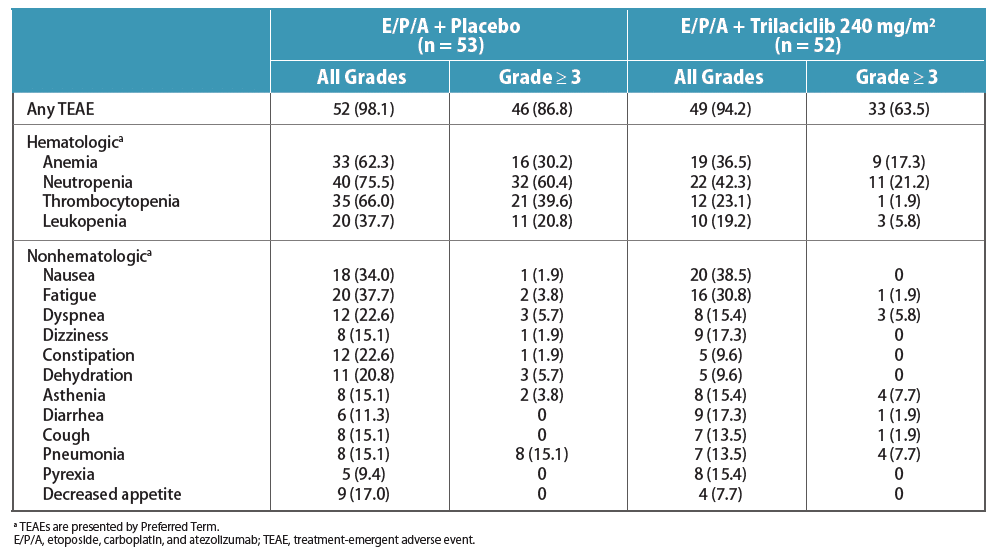
- The most common all-grade and Grade ≥ 3 treatment-emergent AEs (TEAEs) occurring during the induction and maintenance phases are shown in Table 4
- There were fewer Grade ≥ 3 TEAEs with trilaciclib compared with placebo, mostly due to a lower number of Grade ≥ 3 hematologic AEs
- Overall Grade ≥ 3 TEAEs: 63.5% with trilaciclib versus 86.8% with placebo
- Drug-related Grade ≥ 3 TEAEs: 51.9% with trilaciclib versus 75.5% with placebo
- 16 patients had TEAEs considered related to trilaciclib
- The most common TEAEs considered related to trilaciclib were fatigue (9.6%), nausea (7.7%), and anemia and infusion-related reaction (5.8% each); most of these were low grade, with the exception of Grade 3 fatigue (1.9%)
- 12 (23.1%) patients in the trilaciclib arm and 11 (20.8%) in the placebo arm had atezolizumab AEs of special interest, which were mostly immune-related
- Serious TEAEs were reported in 32.7% of patients treated with trilaciclib and 47.2% of patients treated with placebo; 1 (1.9%) serious TEAE (deep vein thrombosis) was considered possibly related to trilaciclib
- In the trilaciclib treatment group, there were 2 deaths due to TEAEs: hemoptysis (n = 1) and pneumonia (n = 1), both considered unrelated to trilaciclib
Source: G1 Therapeutics
Health-Related Quality of Life
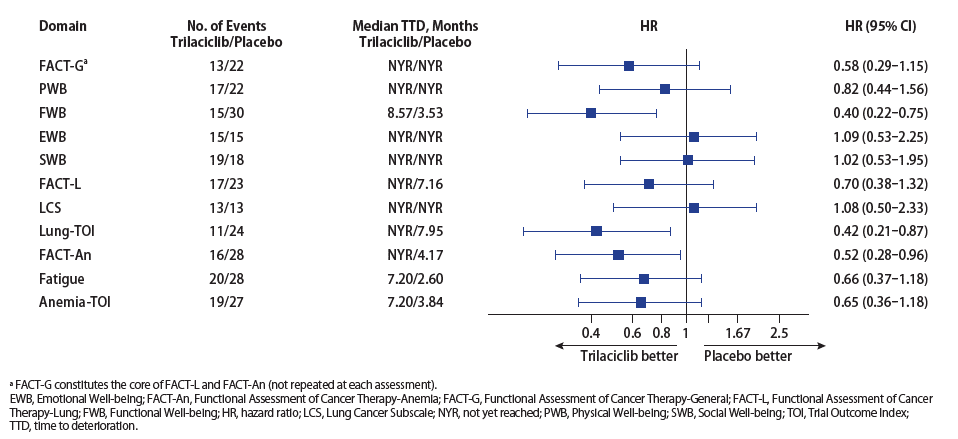
- Enrolled patients had a moderate level of functioning and were moderately symptomatic at baseline as measured by the validated FACT-L and FACT-An instruments
- Trilaciclib improved the patient experience by delaying deterioration of patient functioning and symptom measures over time, compared with placebo. Overall, the benefit of trilaciclib was seen with functional well-being, quality of life measures specific for patients with lung cancer, and symptoms and impact of fatigue, as well as with symptoms and effects on physical and functional well-being due to anemia (Figure 4)
- Statistically significant differences between the trilaciclib and placebo treatment groups were observed for Functional Well-being, Lung-Trial Outcome Index, and FACT-An total score (Figure 4)
Source: G1 Therapeutics
Antitumor Efficacy
- Investigator-assessed objective response rate (ORR) was comparable between trilaciclib (56.0%) and placebo (63.5%) treatment groups
- The clinical benefit rate, including patients with confirmed complete response, confirmed partial response, or stable disease for at least 5 weeks from cycle 1, day 1, was 96.0% with trilaciclib compared with 90.4% with placebo
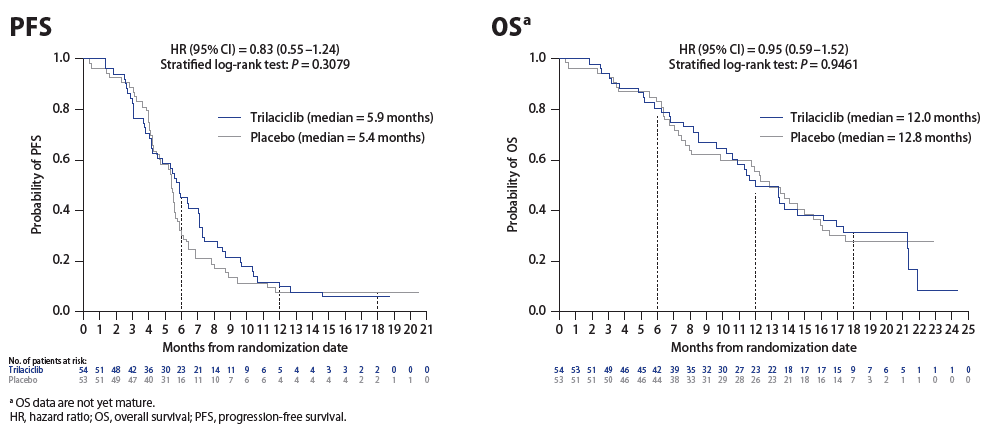
- Median progression-free survival (PFS) was 5.9 (95% CI, 4.2–7.1) months for trilaciclib compared with 5.4 (95% CI, 4.3–5.7) months for placebo (hazard ratio [HR], 0.83; P = 0.3079; Figure 5)
- With a median follow-up of 10.9 months (range, 0.0–24.3 months), median overall survival (OS) was 12.0 (95% CI, 9.6–16.2) months for trilaciclib compared with 12.8 (95% CI, 7.9–15.5) months for placebo (HR, 0.95; P = 0.9461; Figure 5)
Flow Cytometry
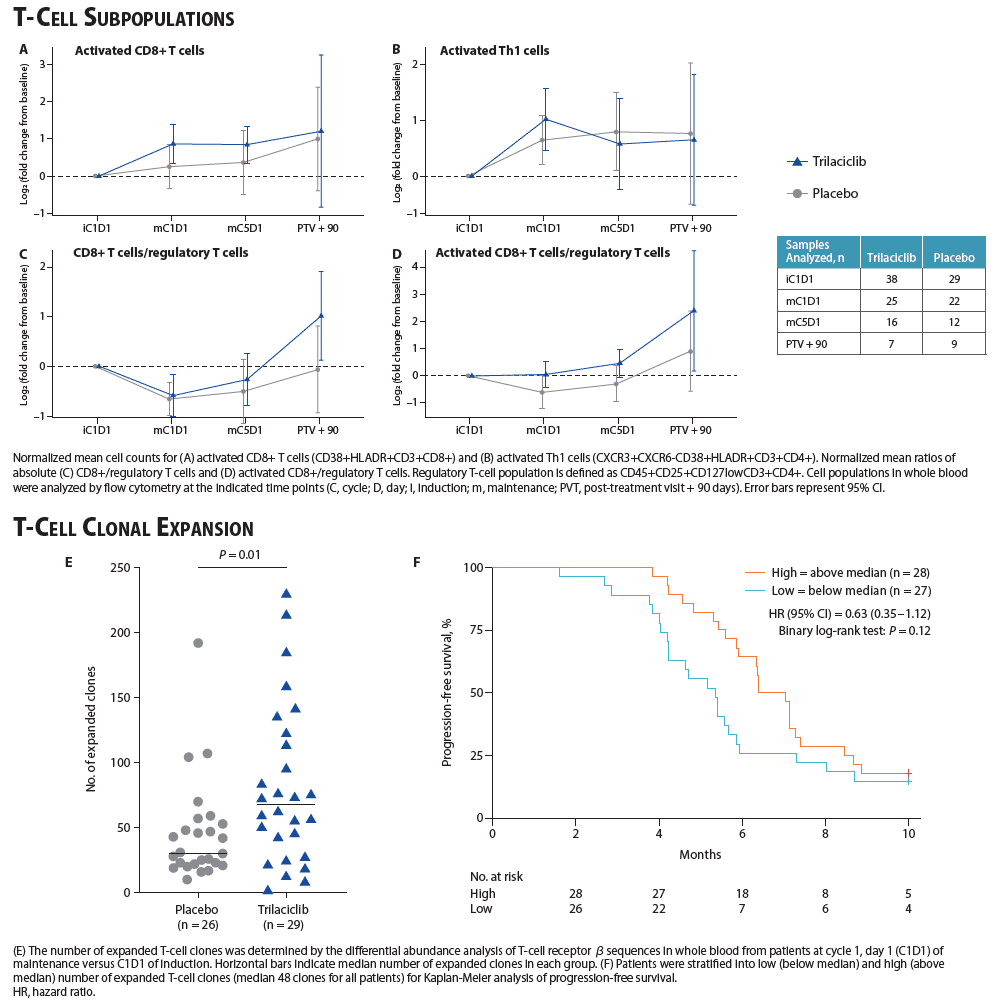
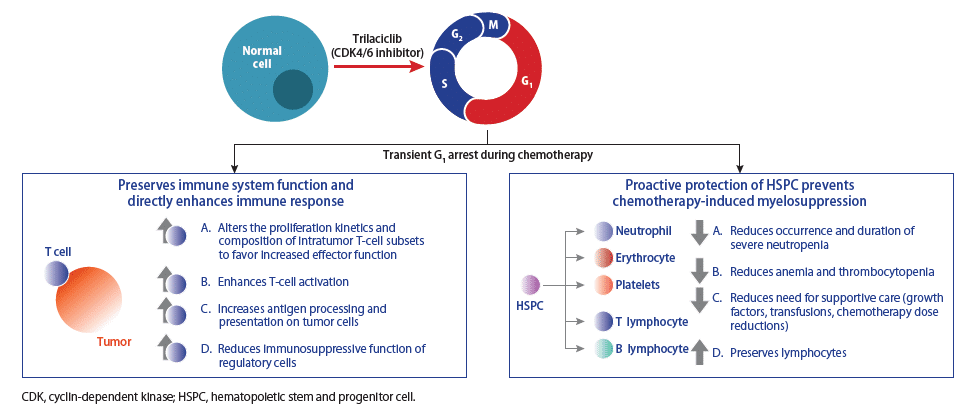
- The addition of trilaciclib to etoposide, carboplatin, and atezolizumab treatment increased the number of circulating activated CD8+ T and Th1 cells during chemotherapy, and increased the ratio of total and activated CD8+ T cells to regulatory T cells in both the induction and maintenance phases of treatment in peripheral blood (Figure 6A–D)
- Patients treated with trilaciclib had significantly higher numbers of expanded T-cell clones than patients treated with placebo (P = 0.01; Figure 6E)
- Regardless of treatment, patients with high levels of T-cell clones had longer PFS (Figure 6F)
Conclusions
- Compared with placebo, trilaciclib makes etoposide, carboplatin, and atezolizumab treatment safer and more tolerable by protecting patients from chemotherapy-induced myelosuppression as evidenced by:
- Effects on neutrophils (statistically significant improvement in primary endpoints of the duration of severe neutropenia, and occurrence of severe neutropenia), RBCs (lower rates of Grade 3/4 anemia and transfusions), and platelets (lower rates of Grade 3/4 thrombocytopenia and transfusions)
- Fewer supportive care requirements
- Fewer chemotherapy dose reductions
- Numerically increased relative dose intensities of etoposide, carboplatin, and atezolizumab
- Improved overall safety profile, primarily due to a reduction in high-grade hematologic AEs attributable to cytotoxic chemotherapy
- Validated patient-reported outcome instruments suggest that the addition of trilaciclib improves the patient experience on chemotherapy
- ORR, PFS, and OS data demonstrate that trilaciclib does not impair chemotherapy/atezolizumab antitumor efficacy
- Flow cytometry data suggest that during treatment with etoposide, carboplatin, and atezolizumab, coadministration of trilaciclib can enhance the T-cell immune response
- These data confirm the myelopreservation benefits of trilaciclib observed in another first-line trial of trilaciclib in combination with etoposide and carboplatin in ES-SCLC (NCT02499770)5 as well as in combination with topotecan in patients previously treated for ES-SCLC (NCT02514447)7
Related Article: New Patient-Reported Outcomes Data shows Trilaciclib Improves Chemotherapy Experience for Patients