As we come to the end of 2020 with the first vaccines by Pfizer/BioNTech and Moderna now approved by the FDA and Health Canada, it seems like an appropriate time to reflect on how Canada has responded to the COVID-19 pandemic to date from a health and innovation standpoint. In this edition of The Takeaway, we look to provide an objective view on the country’s pandemic response, highlighting where we outperformed and where we could have done better. Curriculum below:
Introduction to Pandemics 101: How well has Canada controlled the general spread of COVID-19? Grade: B-
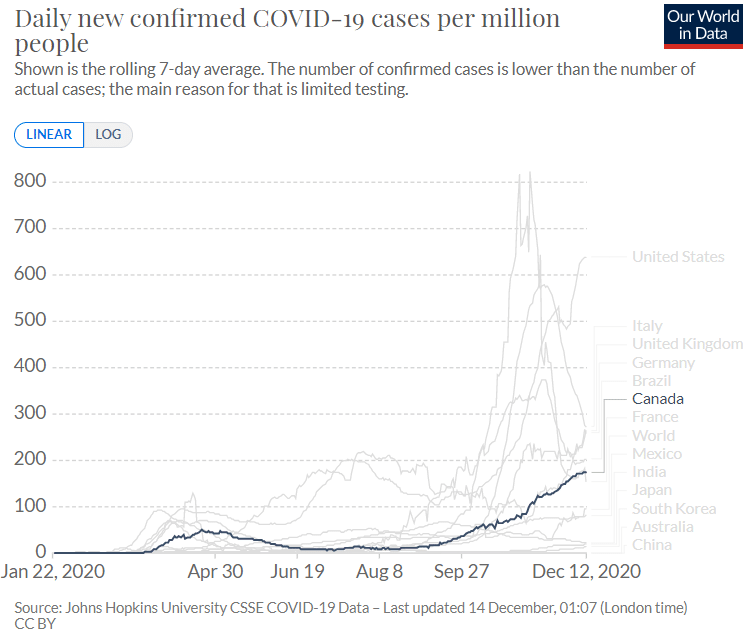
Source: “Our World in Data: Coronavirus Pandemic”, https://ourworldindata.org/coronavirus, accessed: Dec 14, 2020
Infection rates are the most obvious and direct measure of how countries are performing and for Canada, the trends speak for themselves. The country was able to contain the virus fairly well during the first wave as a result of clear and aligned guidance from federal/provincial/municipal public health officials as well as strong buy in and adherence from the general public. However, as we moved into the fall we became complacent to these directives and are now seeing a significant resurgence as a result with daily new infection rates now over 10 times the lowest point in the summer. Many areas across the country are going back into lockdown and while the first vaccine has been approved, it may also provide a false sense of security in the near term while the government works to distribute supply to the wider population. This resurgence has put Canada with a group of countries that clearly do not have the coronavirus under control. In “school terms”, we started off with an A but we got a C in the mid-term exam; hopefully we can recover before the final.
Government Funded R&D 207: How well was public funding allocated to various R&D programs at innovative Canadian companies? Grade: B+
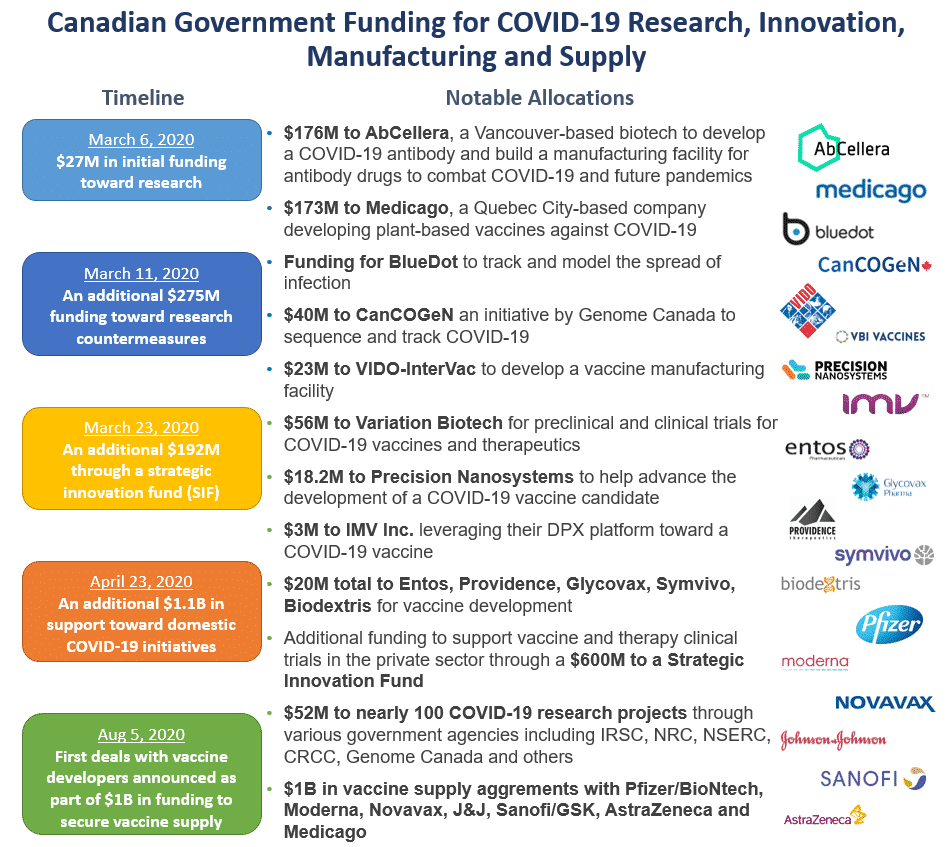
As the country went into lockdown in early March, Canada quickly announced a series of funding programs to support COVID-19 initiatives focussed on detection, tracking, treatment and prevention of the virus. As of the end of November, the government has announced over CAD$2.5B to the private sector toward COVID-19 R&D, manufacturing and supply. Much of the initial funding was provided to domestic companies and leveraged our strong Canadian healthcare infrastructure, but we’ve since secured supply with global pharma/biotech players as vaccines have progressed.
The government’s strategy early on was to distribute a majority of funding evenly between therapeutics and vaccines. This was a good strategy to start, as it was unclear which of the two modalities would ultimately enable us to best combat the virus. Recall when the pandemic began, many experts had best case scenarios for a potential vaccine approval in 2021 (again, at the time no vaccines had been developed for other coronaviruses SARS and MERS). So investment in COVID-19 therapeutics early on was well justified to bridge a potential delay in vaccine discovery. However, given how quickly vaccines have been developed and how effective they’ve proven to be, the role of therapeutics will now be a much shorter intermediate step while the vaccines are rolled out. Funding has also been fairly balanced across the technologies by maturity, with investment in high potential/less proven approaches (such as mRNA vaccines prior to their approval) as well as more established approaches (such viral vector and protein-based approaches that have been used for other infectious diseases). Again, a good strategy by government to spread their eggs across different baskets.
On similar lines, our government, led by advice from the vaccine task force (covered in the next section), did exceptionally well to secure abundant vaccine supply for Canadians. Rather than place a bet on a single vaccine being successful, the strategy was to secure ample supply of all 7 vaccines with the highest probability of success using over CAD$1B in funding. The minimum agreement Canada signed was for 20 million doses, in case only one or two ended up being successful. The government took significant risk with each contract as these agreements were mostly cash up-front regardless of the success of each program but they chose not to compromise the future health and safety of its citizens. This was absolutely the right decision and this was funding well spent, regardless of the outcomes.
One area where the government could have done better was to allocate capital more evenly to more companies earlier on. Here we mean tens of companies with validated platforms and scientific rationale against COVID-19, not hundreds of companies that would dilute the impact of this funding. Specifically, CAD$176M was provided to AbCellera early in the pandemic, prior to the formation of the various task forces, for R&D and to build an antibody manufacturing facility for COVID-19 and future pandemics. While we do recognize the high urgency to kick start impactful COVID-19 initiatives at the time, this was a significant amount to commit toward a single modality. While Eli Lilly/AbCellera’s antibody bamlanivimab has been approved, this pandemic has shown us that the solution to future pandemics is more likely to be multi-modal. Therapies approved by the FDA have consisted of small molecule antivirals – remdesivir and favipiravir; a small molecule JAK1/2 inhibitor – baricitinib; antibodies – bamlanivimab and REGN-COV2; and others. This non-dilutive (and mostly non-repayable) funding certainly provided AbCellera with momentum heading into their US$105M Series B and hugely successful IPO. To be clear, we do not question AbCellera’s platform or technology and we are thrilled to see another major success story come out of the Canadian ecosystem, but context matters. The one-time funding of AbCellera in 2020 represents more funding than the government had provided to the entire innovative Canadian healthcare sector over an 8 year period through the Venture Capital Action Plan (VCAP) and Venture Capital Catalyst Initiative (VCCI) combined. It is hard to imagine and difficult to understate the impact that this kind of engagement by government in Canada’s innovative healthcare sector would have had, had it been made a decade ago. Hopefully the road forward will see the government prioritize the innovative healthcare sector as an economic restart enabler in the same way that they are doing with their clean tech initiatives.
Leveraging Homegrown Expertise 218: How well has Canada leveraged its experts and thought leaders in its response to COVID-19? Grade: A+
In May and June, the federal government established three very important task forces that played key guiding roles in Canada’s COVID-19 strategy. These were:
- The COVID-19 Immunity Task Force, to map the scope of coronavirus infection in Canada
- The COVID-19 Therapeutics Task Force, to provide expert advice on COVID-19 therapeutics research and development
- The COVID-19 Vaccine Task Force, to prioritize and evaluate vaccine candidates and secure access
These task forces brought together the best healthcare expertise and knowledge that Canada has built here at home. They included representatives from Canada’s world-renowned research institutions (CIFAR, IRCM + advisors at many others) and academia (University of Toronto, University of British Columbia, McGill University, University of Saskatchewan, University of Alberta, Dalhousie University + advisors at many others), industry (Zymeworks, Sanofi Pasteur, Vanrx), industry organizations and accelerators (LifeSciences BC, adMare, TIAP), and healthcare investors (Lumira Ventures, Teralys, Amplitude, Sofinnova).
These task forces were instrumental in providing guidance and direction to the government to inform COVID-19 strategy and execution. The vaccine supply agreements that Canada has established were direct recommendations from the vaccine task force, and we commend Minister Bains, Minister Anand and Minister Hajdu for closely following their advice. As of December 9th, Canada has secured 429 million vaccine doses from the seven leading vaccine players (Pfizer/BioNTech, Moderna, AstraZeneca/Oxford, Janssen/Johnson & Johnson, Medicago/GlaxoSmithKline, Novavax, and Sanofi/GlaxoSmithKline), more than any other country on a per capita basis.
We are well aware of the significant effort and time commitment provided by members of the various task forces and would like to sincerely thank them all for their contributions.
Canadian Healthcare Ecosystem Response to Pandemics 315: How has the Canadian healthcare ecosystem supported the search for therapies and vaccines? Score: B+
While Canadian researchers have not yet directly produced an approved therapeutic or vaccine against COVID-19, it is important to acknowledge our significant contributions to the global fight against COVID-19.
First and foremost, the Pfizer/BioNTech vaccine uses a lipid nanoparticle (LNP) formulation developed by Dr. Thomas Madden and his team at B.C.-based Acuitas. LNP is an essential component of the Pfizer vaccine to efficiently deliver mRNA into cells to trigger an immune response.
Canadian researchers and technologies have also contributed to the vaccines that are currently in the clinic. The CanSino vaccine, one of the first to enter the clinic, utilizes Adenovirus Type 5 (AdV 5) first developed by Canadian researcher, Frank Graham nearly 40 years ago. CanSino was collaborating with the National Research Council early on in the process . Medicago is presently the only Canadian company with a vaccine in human trials however there are a handful of other vaccine trials being conducted in Canada (NCT04439045, NCT04442048) and additional vaccine programs in the preclinical stage like Variation Biotech’s VLP-based vaccine candidate and IMV’s DPX-COVID-19 vaccine candidate.
On the therapeutics side, as noted Eli Lilly’s bamlanivimab was discovered using AbCellera’s antibody discovery platform and has now been approved by the FDA and Health Canada. Bamlanivimab was shown to reduce the hospitalization rate for mild/moderate COVID-19 patients that are considered high-risk to progress. Remdesivir is manufactured in Alberta, and researchers at the University of Alberta have discovered a novel, second mechanism of action by which this drug acts against SARS-CoV-2. As of December 14th there are 69 COVID-19 therapeutics trials being conducted in Canada (https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/list-authorized-trials.html). These trials are being led by both academia/research institutions as well as the private sector across a number of different therapeutic modalities. Both Canadian companies, such as Edesa Biotech and Appili Therapeutics, and international pharma/biotechs like Novartis, Gilead, Roche, Merck, Sanofi and GSK, are all taking advantage of Canada’s strong clinical trial infrastructure.
All said, Canada has clearly left its mark on the global pandemic response.
Domestic Policy 403: How well prepared are we for the next phase of the pandemic? Grade: TBD
While the vaccines are just starting to be rolled out, it is a relief to end the year knowing that the end is within sight. We have been able to battle the pandemic using homegrown technologies and expertise, we have mobilized our healthcare ecosystem toward advancing potential treatments, and made our mark on global scientific effort to find therapies and vaccines. The Canadian government has recently announced that vaccines will be free for Canadians and any excess vaccine stock will be donated to developing countries in need. Overall, the country should be commended for prioritizing its citizens, while still making commitments to support the fight in developing countries (also of note, Canada has committed an addition CAD$485M to the global ACT-Accelerator program).
That said, it will take some time for vaccines to be rolled out and we still have lots of work to do to get the virus back in control in the interim. With much still left to be done, we’ll leave the last grade for the end of 2021.
The Takeaway
- Canada as a nation is currently failing to get the virus under control; we must do better.
- Public spending into domestic R&D was substantial and generally well allocated. The only area for improvement would have been to distribute early funding to a broader group of companies and perhaps to have had all this facilitated by a national strategy with respect to domestic healthcare innovation.
- Canada did very well to bring together homegrown knowledge and expertise through the COVID-19 task forces.
- Canada made and continues to make significant contributions in the global effort to find effective therapeutics and vaccines against COVID-19.
The Takeaway is a periodic blog by the team at Lumira Ventures that covers themes and trends within the fast-growing Canadian healthcare innovation ecosystem. Please note that the views and opinions expressed in this article are those of Lumira Ventures and do not reflect the official policy or position of any of our investors, co-investors, or external partners.
Author: Nikhil Thatte, Principal