- Plans to submit U.S. and European marketing applications for trilaciclib following regulatory feedback
- First clinical data on oral SERD G1T48 expected in 3Q19
- Management to host webcast and conference call today at 4:30 p.m. ET
RESEARCH TRIANGLE PARK, N.C., May 09, 2019 (GLOBE NEWSWIRE) — G1 Therapeutics, Inc. (Nasdaq: GTHX), a clinical-stage oncology company, today provided a corporate and financial update for the first quarter ended March 31, 2019.
“Following meetings with regulatory authorities, we have clarity on a path to submitting marketing applications in the U.S. and Europe for trilaciclib based on existing data from our three trials in small cell lung cancer patients,” said Mark Velleca, M.D., Ph.D., Chief Executive Officer. “Our goal is to make trilaciclib available to patients across the globe as quickly as possible and we are encouraged with regulators’ understanding of this new approach to protecting patients from the damaging effects of chemotherapy.”
Raj Malik, M.D., Chief Medical Officer added, “Trilaciclib is the first in a deep pipeline of clinical-stage programs with near-term data readouts expected. We anticipate presenting new data on our next two programs – lerociclib and G1T48 – later this year. Based on promising early results in our Phase 1 clinical trial of G1T48 in ER+ breast cancer, we plan to present proof-of-concept data in the third quarter.”
Clinical, Operational and Executive Team Updates
- Plan to submit U.S. and European regulatory filings for trilaciclib: The company announced plans to submit marketing applications in the U.S. and Europe for trilaciclib for myelopreservation in small cell lung cancer (SCLC) based on written feedback from its end-of-Phase 2 meeting with the U.S. Food and Drug Administration (FDA) and discussions with European regulatory authorities. G1 intends to file a New Drug Application (NDA) with the FDA in 2020 and submit a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) subsequent to an NDA filing. Full press release available here.
- Executive team change: Jennifer Moses, who has been with the company for four years and most recently served as Vice President, Finance, has been appointed Chief Financial Officer. Barclay “Buck” Phillips, who had served as CFO and Senior Vice President, Corporate Development since 2017, departed the company to pursue other interests and opportunities.
- First investor day presentation: The G1 management team provided a comprehensive overview of the company’s three clinical development programs and outlined the commercialization strategy for trilaciclib. External experts Jeffrey Crawford, M.D., Co-director, Solid Tumor Therapeutics Program, Duke Cancer Institute, and Lowell Hart, M.D., Scientific Director of Research, Florida Cancer Specialists and trilaciclib clinical trial investigator, discussed chemotherapy-induced myelosuppression and trilaciclib’s potential to protect the bone marrow from damage by chemotherapy and improve patient outcomes. The webcast is available on the G1 website here.
First Quarter 2019 Financial Highlights
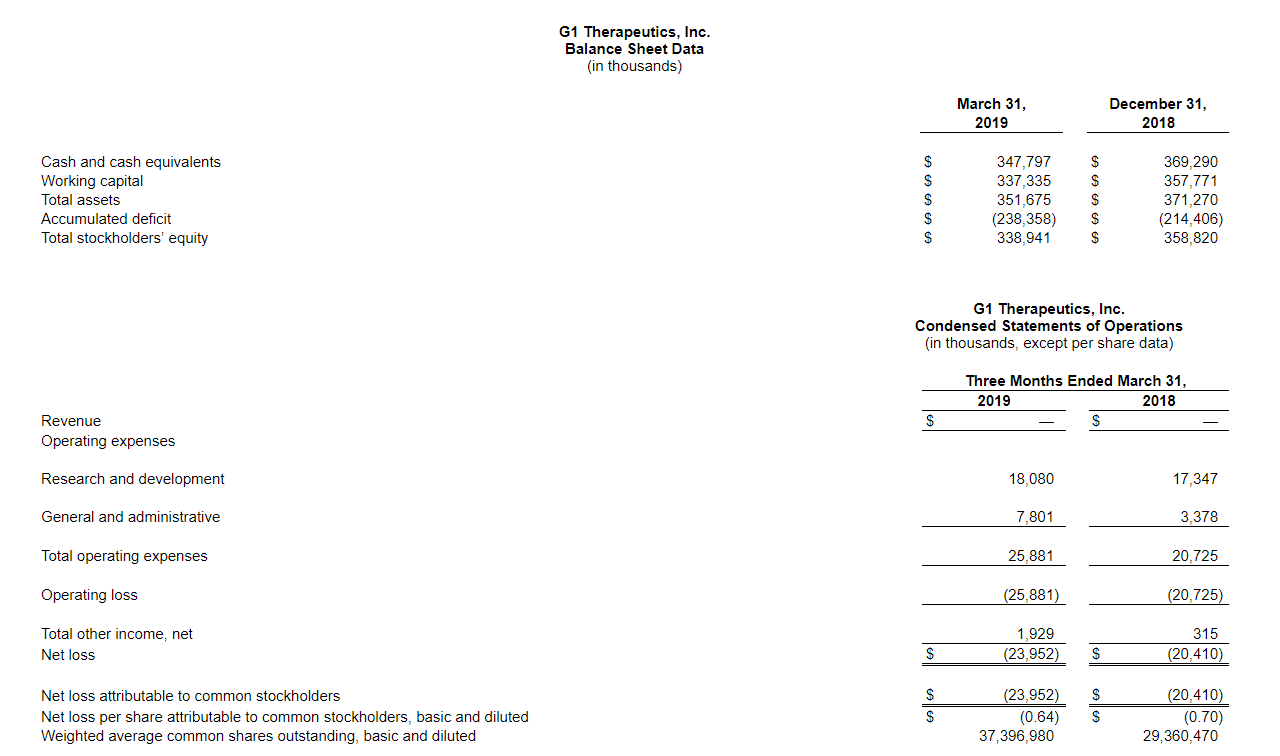
- Cash Position: Cash, cash equivalents and short-term investments totaled $347.8 million as of March 31, 2019, compared to $369.3 million as of December 31, 2018.
- Operating Expenses: Operating expenses were $25.9 million for the first quarter of 2019, compared to $20.7 million for the first quarter of 2018. GAAP operating expenses include stock-based compensation expense of $3.8 million for the first quarter of 2019, compared to $1.6 million for the first quarter of 2018.
- Research and Development Expenses: Research and development (R&D) expenses for the first quarter of 2019 were $18.1 million, compared to $17.3 million for the first quarter of 2018. The increase in expense was primarily due to an increase in clinical program costs and personnel costs due to additional headcount.
- General and Administrative Expenses: General and administrative (G&A) expenses for the first quarter of 2019 were $7.8 million, compared to $3.4 million for the first quarter of 2018. The increase in expense was largely due to an increase in compensation due to headcount increase, increase in pre-commercialization activities and an increase in professional fees and other administrative costs necessary to support our operations as a public company.
- Net Loss: G1 reported a net loss of $24.0 million for the first quarter of 2019, compared to $20.4 million for the first quarter of 2018.
Anticipated Milestones for 2019
- Expect to complete pre-NDA meeting with the FDA.
- Report additional data from all four randomized Phase 2 trilaciclib clinical trials.
- Present proof-of-concept data from the Phase 1 clinical trial of G1T48, an oral selective estrogen receptor degrader (SERD), in ER+ breast cancer in Q3 2019.
- Present preliminary dose-escalation data from the Phase 1b clinical trial of lerociclib/Tagrisso® (osimertinib) in non-small cell lung cancer in Q3 2019.
- Present additional data from the Phase 1b clinical trial of lerociclib/Faslodex® (fulvestrant) in ER+, HER2- breast cancer in Q4 2019.
Webcast and Conference Call
The management team will host a webcast and conference call at 4:30 p.m. ET today to provide a corporate and financial update for the first quarter of 2019 ended March 31, 2019. The live call may be accessed by dialing 866-763-6020 (domestic) or 210-874-7713 (international) and entering the conference code:7988598. A live and archived webcast will be available on the Events & Presentations page of the company’s website: www.g1therapeutics.com. The webcast will be archived on the same page for 90 days following the event.
About G1 Therapeutics
G1 Therapeutics, Inc. is a clinical-stage biopharmaceutical company focused on the discovery, development, and delivery of innovative therapies that improve the lives of those affected by cancer. The company is advancing three clinical-stage programs. Trilaciclib and lerociclib are designed to enable more effective combination treatment strategies and improve patient outcomes across multiple oncology indications. G1T48 is a potential best-in-class oral selective estrogen receptor degrader (SERD) for the treatment of ER+ breast cancer. G1 also has an active discovery program focused on cyclin-dependent kinase targets.
G1 is based in Research Triangle Park, N.C. For additional information, please visit www.g1therapeutics.com and follow us on Twitter @G1Therapeutics.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “will,” “expect,” “plan,” “anticipate,” “estimate,” “intend” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Forward-looking statements in this news release include, but are not limited to, the therapeutic potential of trilaciclib, lerociclib and G1T48 and the timing for next steps with regard to the trilaciclib marketing applications, and are based on the Company’s expectations and assumptions as of the date of this press release. Each of these forward-looking statements involves risks and uncertainties. Factors that may cause the Company’s actual results to differ from those expressed or implied in the forward-looking statements in this press release are discussed in the Company’s filings with the U.S. Securities and Exchange Commission, including the “Risk Factors” sections contained therein and include, but are not limited to, the Company’s ability to complete clinical trials for, obtain approvals for and commercialize any of its product candidates; the Company’s initial success in ongoing clinical trials may not be indicative of results obtained when these trials are completed or in later stage trials; the inherent uncertainties associated with developing new products or technologies and operating as a development-stage company; the Company’s development of a CDK4/6 inhibitor to reduce chemotherapy-induced myelosuppression is novel, unproven and rapidly evolving and may never lead to a marketable product; and market conditions. Except as required by law, the Company assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available.
Contact:
Jeff Macdonald
Head of Investor and Public Relations
919-213-9835
jmacdonald@g1therapeutics.com
Related Article: G1 Therapeutics Announces Closing of Offering of Common Stock
