Study Conclusions
Yesterday, Cardiac Dimensions met its primary endpoint. Results from the REDUCE FMR clinical study were published in the Journal of American College of Cardiology: Heart Failure.
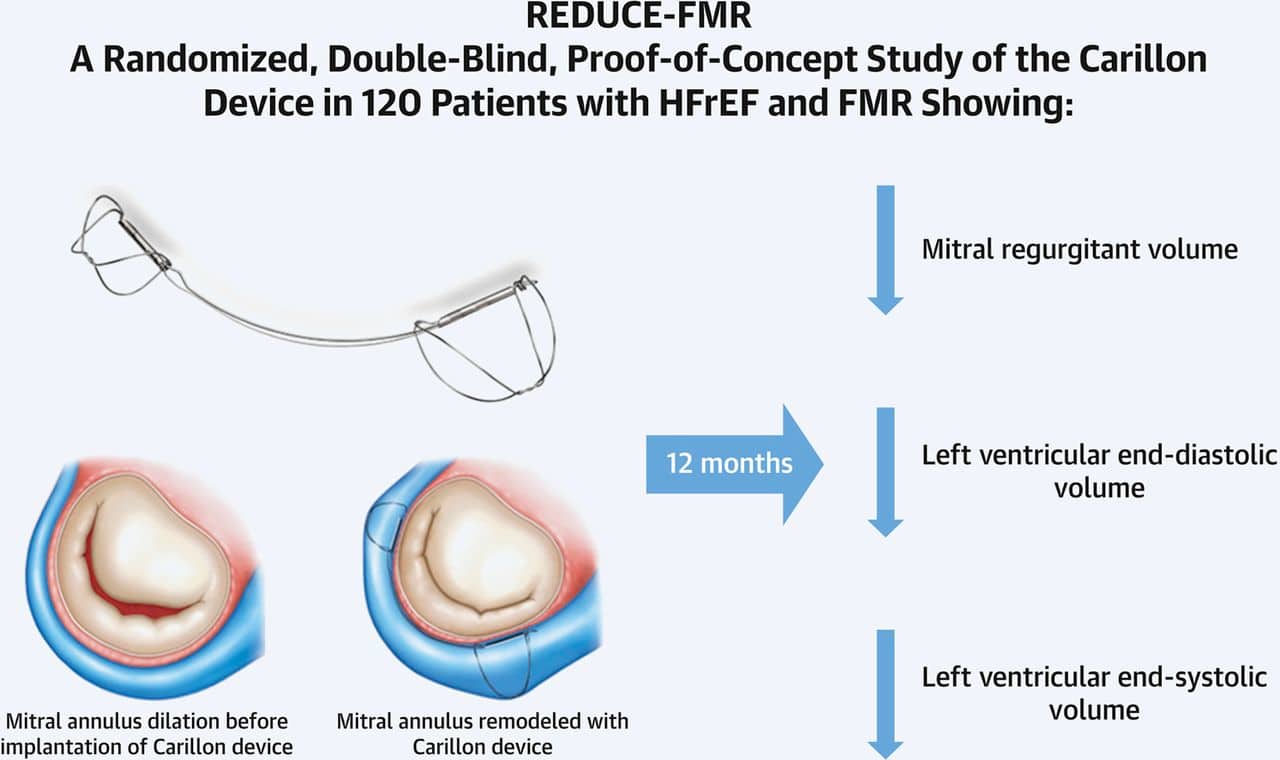
In this first blinded and sham-controlled randomized controlled trial of a percutaneous heart valve therapy, REDUCE-FMR, has demonstrated that a low-risk transvenous approach to mitral annuloplasty can successfully and safely reduce FMR. This reduction is associated with reverse LV remodeling. The simplicity of this approach is supported by the study being largely done by centers previously unfamiliar with the technology. Other advantages include the right-sided approach, avoidance of trans-septal puncture and the fact that Carillon® device placement does not preclude any future mitral valve treatment if needed later. Studies are now underway to assess the effect of this approach on clinical outcomes.
Source: Journal of American College of Cardiology: Heart Failure (September 2019)
Related Article: New Data Confirms Cardiac Dimensions’ Carillon System Provides Acute Hemodynamic Efficacy in Patients with Functional Mitral Regurgitation
Key Findings:
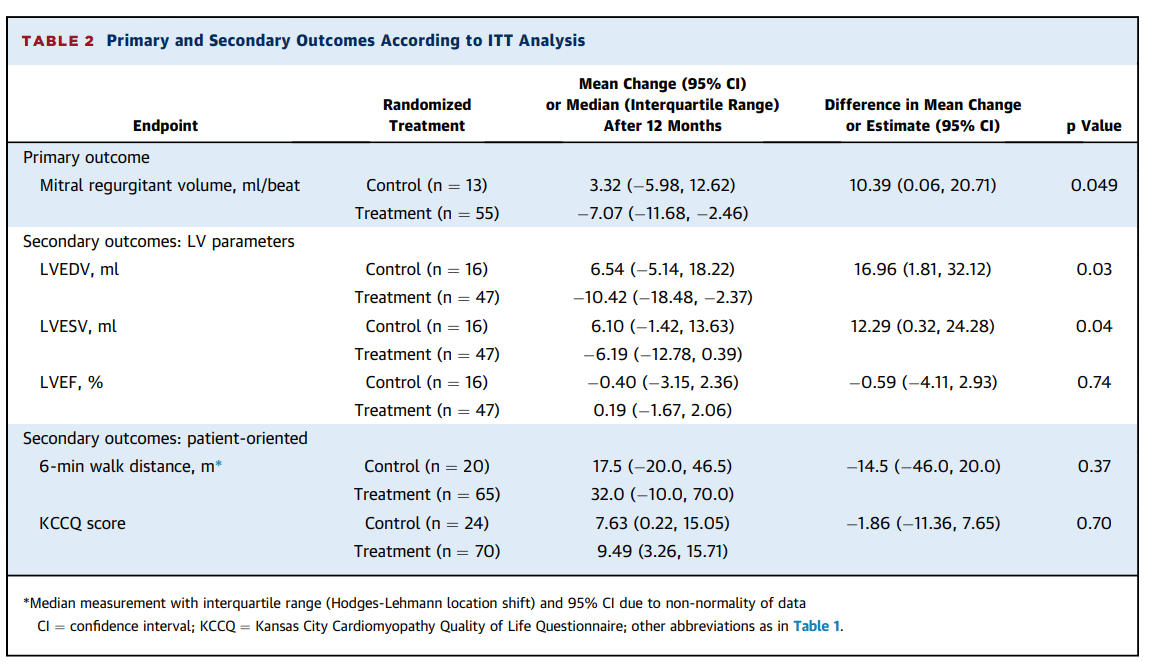
- Treatment with the Carillon® device resulted in a statistically significant reduction of mitral regurgitant volume vs. the control group (decrease of 7.1 mL/beat vs. an increase of 3.3 mL/beat; p=0.049)
- Treatment with the Carillon® device induced a significant reduction of left ventricular volumes vs. the control group, (LVEDV: decrease of 10.4 mL vs. an increase of 6.5 mL; p=0.03 and LVESV: decrease of 6.2 mL vs. an increase of 6.1 mL; p=0.04)
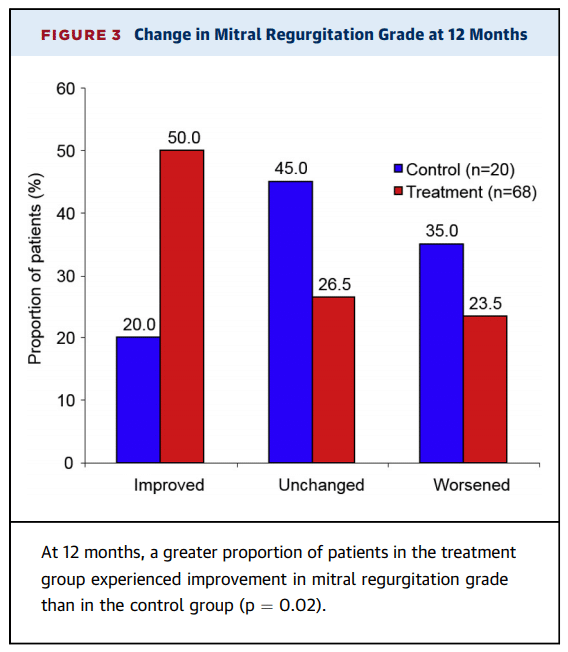
- Significant improvements in regurgitant volume and LVEDV were observed across all MR grades with the absolute improvement in both measures the greatest in patients with MR grades 3+ and 4+ after 12 months (reduction of mitral regurgitant volume: 12.8 ± 21.6 (n=15), change in LVEDV: -26.9 ± 16.3 (n=13) and change in LVESV: -17.5 ± 18.9 (n=13))
- Patients treated with the Carillon® device had a significant improvement in 6-minute walk distance at 12 months compared with baseline (p=0.002) whereas patients in the control group did not (p=0.29)
- Patients in the Carillon® treatment group had a significant improvement in NYHA class at 12 months compared with baseline (p=0.002) whereas patients allocated to the control group did not (p=0.75)
- The Carillon® device showed a positive safety profile with a similar incidence of major adverse events between groups through the follow-up period
- Treatment with the Carillon® device enabled patients to experience 48% fewer repeat (>1) heart failure hospitalizations during follow-up (11% v 21%, p=0.23).
Source: Journal of American College of Cardiology: Heart Failure (September 2019)
About the Study
The Carillon® mitral contour system, a mitral annuloplasty device delivered percutaneously to the coronary sinus, is designed to reduce the mitral annular dimension by virtue of the close anatomic relationship between the coronary sinus and posterior mitral annulus. The REDUCE-FMR (Carillon® Mitral Contour System for Reducing Functional Mitral Regurgitation) study was the first sham-controlled randomized trial of any catheter-based therapy for patients with valvular heart disease. The aim was to evaluate the effects of the Carillon® device on FMR severity and LV remodeling.
Study Procedures
After undergoing screening for clinical eligibility, patients provided informed written consent. They then underwent quantitative transthoracic echocardiography by appropriately trained local echocardiographers for the assessment of mitral regurgitation and LV structure and function. After review by the local investigator, with the support of the local heart valve disciplinary team according to local practice, a decision was made about the patient’s suitability for the REDUCE-FMR study.
At a subsequent study visit, patients underwent a 6-minute walk test, submitted blood tests for renal function and natriuretic peptides, and completed a Kansas City Cardiomyopathy Quality of Life Questionnaire (KCCQ). Following this, the patients were taken to the cardiac catheterization laboratory, where they were put under either general anesthesia (n=47) or conscious sedation, using headphones and blindfolds (n=73) as required to maintain blinding. Patients underwent coronary angiography through radial or femoral access. A 10-F sheath was inserted into the right internal jugular vein, and a Carillon® delivery catheter was used to engage the coronary sinus.
Quantitative venous angiography was performed. If the venous dimensions were suitable in diameter and length for a Carillon® device, randomization was performed. In patients randomized to sham control, the procedure was terminated and the sheaths withdrawn. In patients randomized to device implantation, an appropriately sized device was inserted into the delivery catheter and deployed. The distal anchor was unsheathed and locked in a suitable segment of the great cardiac vein. Tension was applied, and the proximal anchor was deployed in the desired location if left coronary angiography had confirmed no impingement of or obstruction to flow in the circumflex coronary artery or its branches.
Subsequently, patients underwent echocardiographic and clinical follow-up at 1, 6, and 12 months after the procedure. All echocardiograms were interpreted according to established guidelines. Specifically to the assessment of mitral regurgitation, a multi-parametric and quantitative approach was used to divide FMR into 4 grades. Trial definitions and the published write-up can be reviewed here.